Copper Complexes of N-Heterocyclic Carbenes (NHCs) in Cross-Coupling Reactions
Cross-coupling reactions are among the most common methods to synthesize valuable, functionalized molecules including natural products, biologically active molecules, conjugated polymers and nanostructures. Transition metal catalysts have been used in numerous carbon–carbon bond formations of which worth mentioning Negishi, Mizoroki–Heck, Stille, Suzuki–Miyaura, Sonogashira and Grignard reagents. Sonogashira coupling occurs between a terminal alkyne (sp) and an aryl- or vinyl halide (sp2) in the presence of a palladium catalyst/copper co-catalyst (Figure 1) and is among the most widely used cross couplings reactions in the drug-development processes. Shown in Figure 1 (right) is an anti-allergic drug (Olopatadine Hydrochloride) that is prepared using the traditional Sonogashira catalyst system, Pd(PPh3)2Cl2 and CuI.
Mechanism
According to a proposed mechanism (Figure 1, left), the actual active site is the Pd catalyst where both oxidative addition of the aryl halide and reductive elimination of the crossed-coupled product occurs. The Cu catalyst (often a cu salt) has been a transmetallating reagent to transfer the alkyne to the Pd center.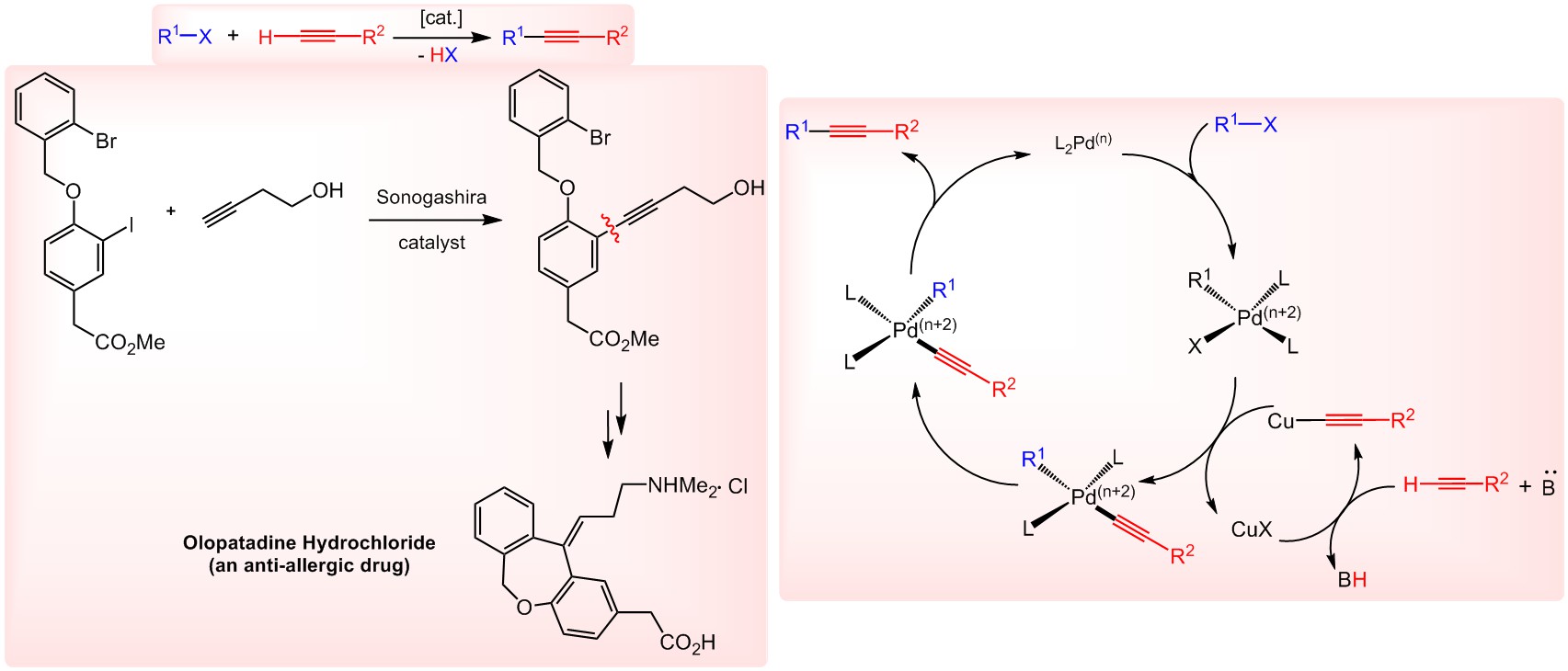
Figure 1.
Cu-NHC Complexes
Palladium has a long-standing history as catalyst in the cross-coupling reactions, however, its significantly high cost has been a disadvantage to scaling-up in the industrial applications. In our lab we attempt to develop cost-effective Cu-based systems that can be used as sole catalysts in the Sonogashira and other cross-coupling reactions. The supporting ligands of our copper complexes are N-heterocyclic carbene ligands (NHCs) owing to their similar electronic properties to that of phosphines which have been extensively used in the cross-coupling reactions with Pd. However, the NHCs provide more flexibility for electronic and steric effects’ modulation and are more feasible to synthesize and handle under ambient conditions. In order to overcome the homo-coupling products’ formation which is a common concern in copper catalysis, our special focus is on the bulkier NHC ligands that would not favor the polymerization of copper acetylide (Figure 2). To fulfill the steric and electronic requirements of the two cycles in the cross-coupling reactions, oxidative addition/reductive elimination and transmetalation, we are interested in and consider both cationic and neutral copper complexes, [Cu(NHC)2]X and [Cu(NHC)Cl], respectively.
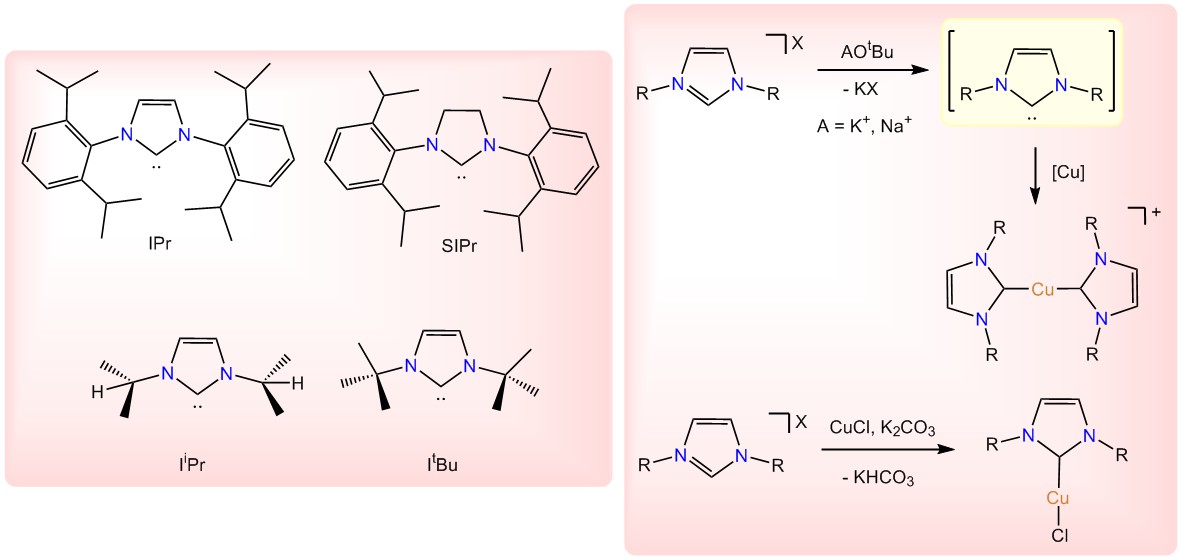
Figure 2.
